Bcl3 Compound Name
Uses of BCl3 are immense. Iodine monochloride nitrogen trichloride dinitrogen trioxide.

Hybridization Of Bcl3 Hybridization Of Boron In Bcl3
Name the following compounds.
Bcl3 compound name. Its steric number is also said to be 3. Name the following compounds. Phosphorus trichloride appears as a colorless or slightly yellow fuming liquid with a pungent and irritating odor resembling that of hydrochloric acid.
Boron trichloride is a molecule that can be industrially produced by chlorinating boron oxide and carbon directly at 501C. Write formulas for the following compounds. It is a colorless inorganic compound that has a pungent odor and appears as fumes in air.
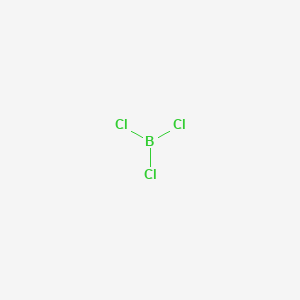
BCl3 is a trigonal planar molecule like the other boron trihalides and has a bond length of 175pm. Very toxic by inhalation ingestion and skin absorption. Boron trichloride BCl3 CID 25135 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information.
Name the compound BaO. Name the following covalent compound. How many valence electrons do most elements need to become stable.
Write the correct formula for. A step-by-step explanation of how to draw the BCl3 Lewis Dot Structure Boron trichlorideFor the BCl3 structure use the periodic table to find the total nu. All Chemistry Practice Problems Naming Molecular Compounds Practice Problems.
BCl3 Lewis Structure Molecular Geometry and Hybridization. What would the formula for Boron Chloride be. Name the following covalent compound.
What is the correct name for the molecular compound BCl3 using IUPAC molecular rules Boron trichloride What is the correct formula for the molecular compound N2H4. Corresponding trihalides of boron are produced during the reaction of boron with halogens. A trihalide of Boron BCl 3 consists of a single boron atom and three atoms of Chlorine.
From Wikipedia the free encyclopedia Boron trifluoride is the inorganic compound with the formula BF 3. The name of BCl 3 is boron trichloride. A NaF b Rb2O c BCl3 d H2Se e P4O6 f ICl3.
Answer to Chemical Nomenclature Name the following compounds. This pungent colourless toxic gas forms white fumes in moist air. Name the following covalent compound.
Boron and chlorine are both nonmetals so this is a covalent compound. Name the following covalent compound. In the Lewis structure for BCl3 the central atom Boron will only have six valence electrons.
In BCl 3 molecule boron will be the central atom which contains three bonded atoms but no lone pair of electrons. Learn this topic by watching Naming Molecular Compounds Concept Videos. B2O3 3C 3Cl2 2BCl3 3CO.
It is a useful Lewis acid and a versatile building block for other boron compounds. See full answer below. When naming covalent compounds the.
Causes severe burns to skin eyes and mucous membranes. Note that Boron can have a full outershell with only six valence electrons. Hybridization of BCl3 Boron Trichloride The type of hybridization that occurs in BCl 3 is sp 2 hybridization.
It finds use in various applications including the production of elemental Boron.

Dinitrogen Tetrahydride Ppt Video Online Download
Boron Trichloride Bcl3 Pubchem
What Is The Structure Of Bcl3 Quora
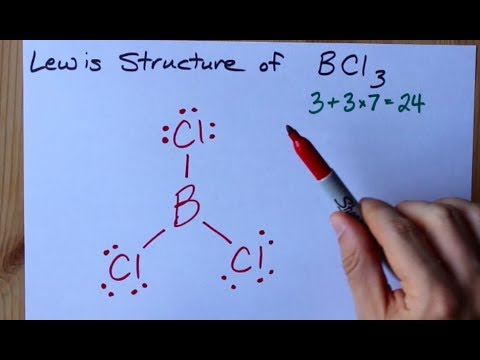
How To Draw The Lewis Structure Of Bcl3 Boron Trichloride Youtube
Boron Trichloride Bcl3 Chemspider
Is This Bcl3 Nh3 A Compound Quora

Bcl3 Lewis Structure How To Draw The Lewis Structure For Bcl3 Youtube

Bcl3 Lewis Structure Molecular Geometry And Hybridization Techiescientist
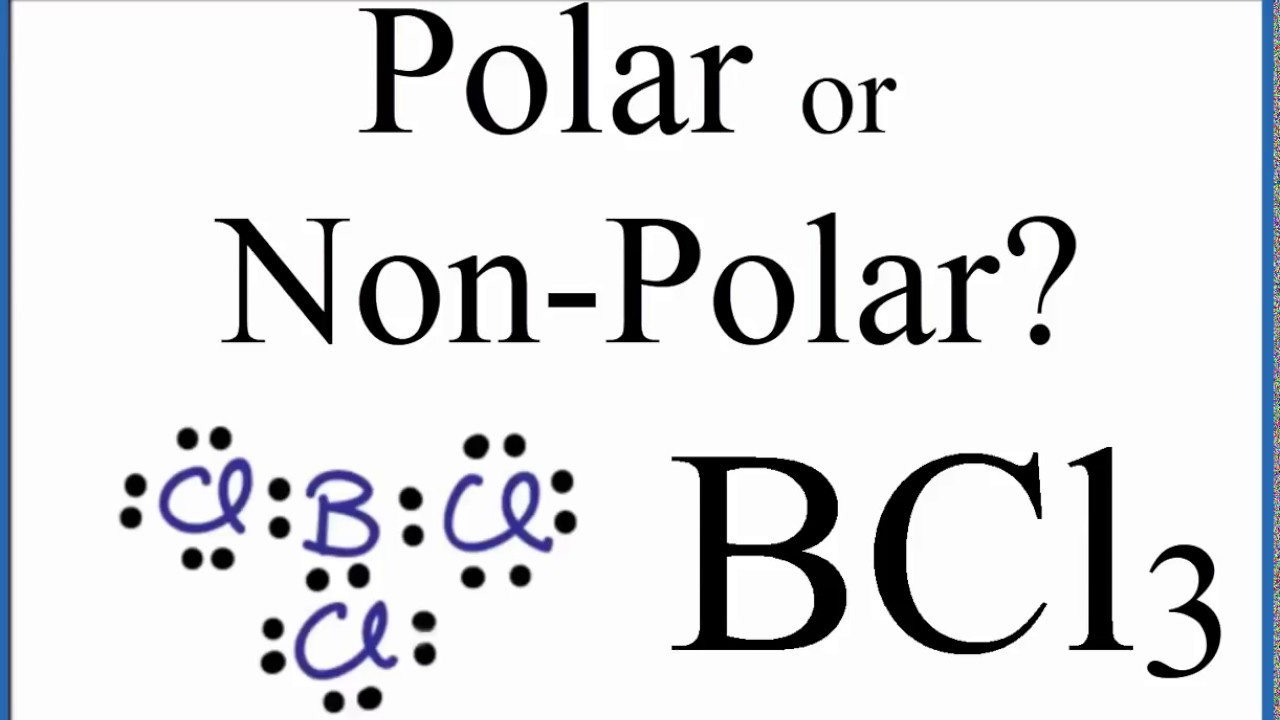
0 Response to "Bcl3 Compound Name"
Post a Comment